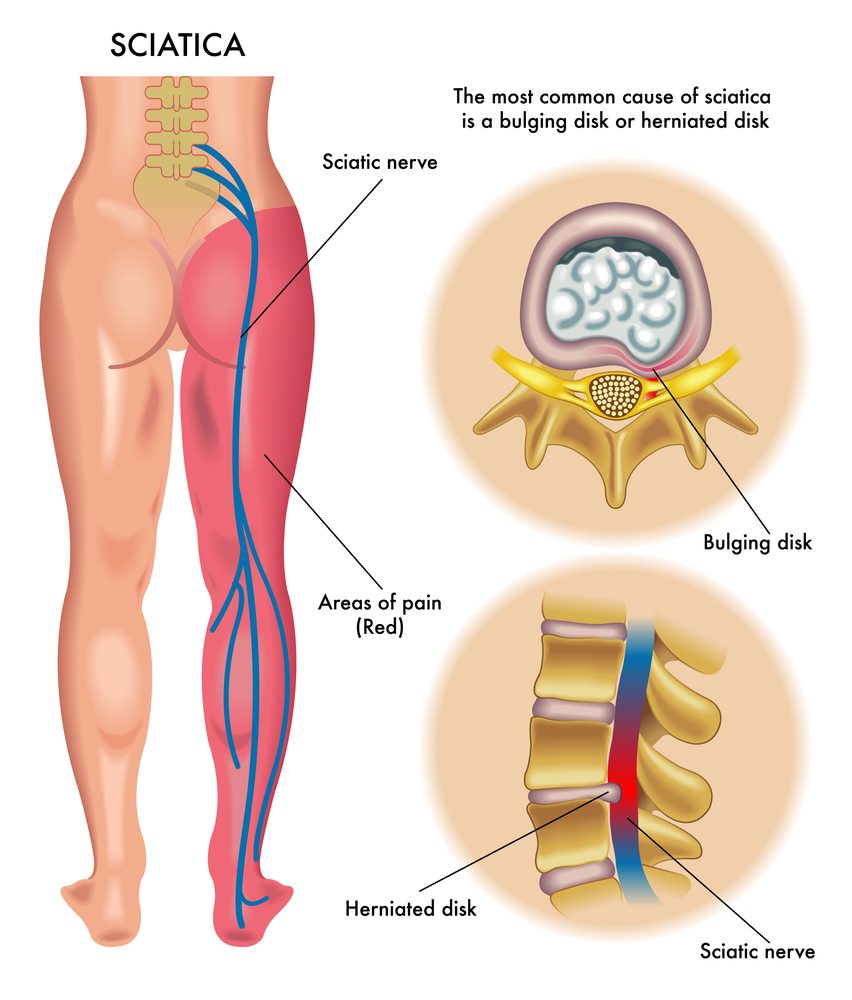
Sciatica
Injectable microtissue helps maintain the function of muscles in rats suffering from sciatic nerves that are severed – Science Daily
Researchers developed the first injection-able microtissue that contains sensory and motor neurons, protected by a protective layer, known as tissue engineered neuromuscular interfaces (TE-NMIs). The TE-NMI neurons serve as the source of axons for muscles in rats that have suffered nerve injuries. They also “babysit” muscles to prevent the degeneration as well as loss of function and damage to the nerve regenerates according to researchers from the Perelman school of Medicine of the University of Pennsylvania. The results were reported in Bioactive Materials.
The TE-NMIs consist of nerve cells enclosed in a hydrogel that is protected and the whole microenvironment of the micro-environment is injected the vicinity of muscles. This “ship inside bottle” method shields neurons and enhances the chance that a higher number of Axons will couple to the muscle and continue to maintain the regenerative pathways.
Researchers cut off the sciatic nerve of rats and then injected them with an TE-NMI or microtissue with no neurons. In the group receiving TE-NMIs the researchers were able to stimulate electrically the nerve stump “babysat” through the TENMI and observe an increase in muscle activity at up to 5 months after the implanted tissue. There was no muscle response in the group that was not treated.
“There many thousands of individuals who go through surgery for nerve injuries each year, and even if surgeons perform the procedure correctly it is impossible to cause axons to regrow more than 1 inch every month. For nerve injuries that affect the arm’s upper or leg, recovery could take years, but the pathway that connects to the muscle as well as the muscle itself will degrade irreparably within six to twelve months of no axons connections which can lead to permanent loss of sensory and motor functionality,” said senior author D. Kacy Cullen, PhD, Associate Professor of Neurosurgery. “By expanding the time frame for the patient’s axons connect to muscles, this research has the potential to enhance the speed of healing for patients, without causing more damage.”
For instance, those suffering from a brachial-plexus injury (a nerve root avulsion, where nerves are separated from spinal cordcould regain elbow functionality however, they will not be able to fully function in their hand. In such cases an neurosurgeon is likely to cut a healthy nerve close to the hand, and redirect it to trigger hand muscles to restore a portion of function, while the nerve grows. The TE-NMIs could perform a better jobwithout needing to cause damage to a patient’s healthy nerve as suggested by researchers.
“Working closely with clinicians from the Penn Nerve Centerfor Neurological Disorders, our team came up with the possibility of a surgical approach that is most beneficial to their patients and the clinic,” said first author Justin Burrell, PhD, an assistant professor within the Department of Neurosurgery and the Institute for Translational Medicine and Therapeutics. “What’s more is that as we continue to investigate and validate our findings, we’ll maintain our collaboration in collaboration with Penn’s Nerve Center to ensure that our research provides them with the equipment they require to provide the highest quality care to patients.”
The study was funded primarily through this study’s funding from the U.S. Department of Defense (W81XWH-16-1-0796, W81XWH-19-1-867) as well as the National Institutes of Health (R44-NS108869 and TL1-TR001880) and the Department of Veteran Affairs (I01-BX003748).
Story Source
Materials supplied by the University of Pennsylvania School of Medicine. Note: The content could be edited to improve length and style.
The