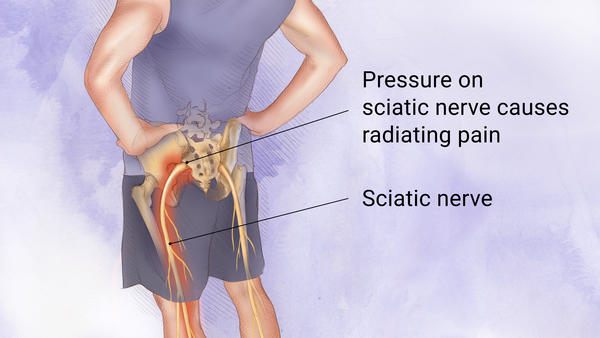
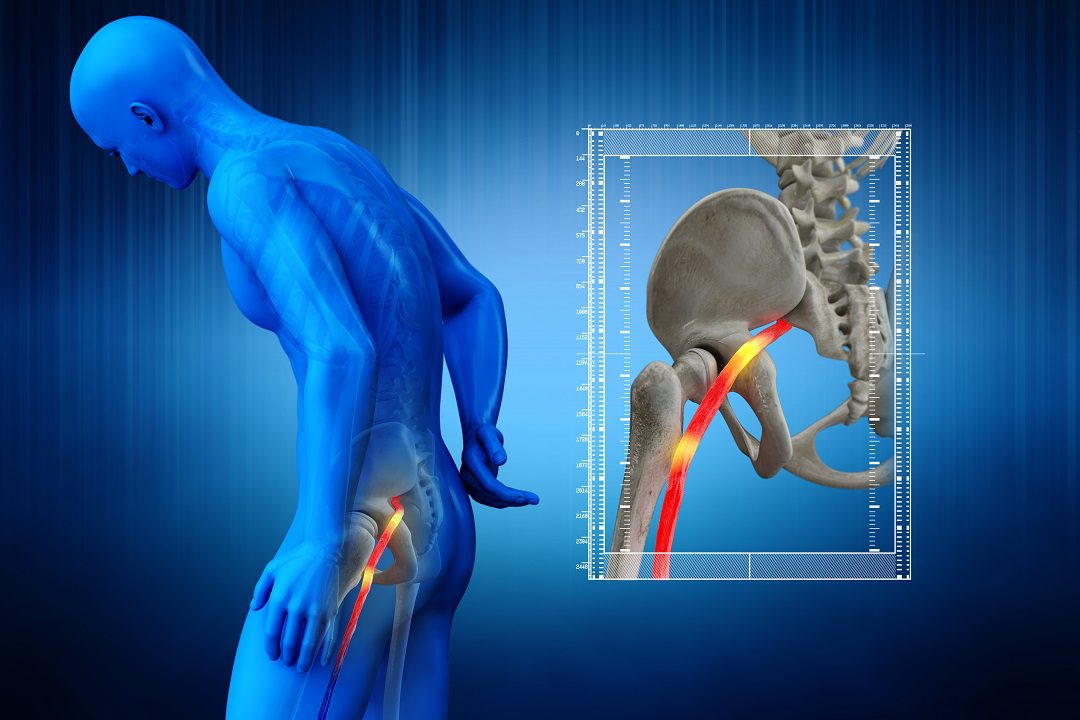
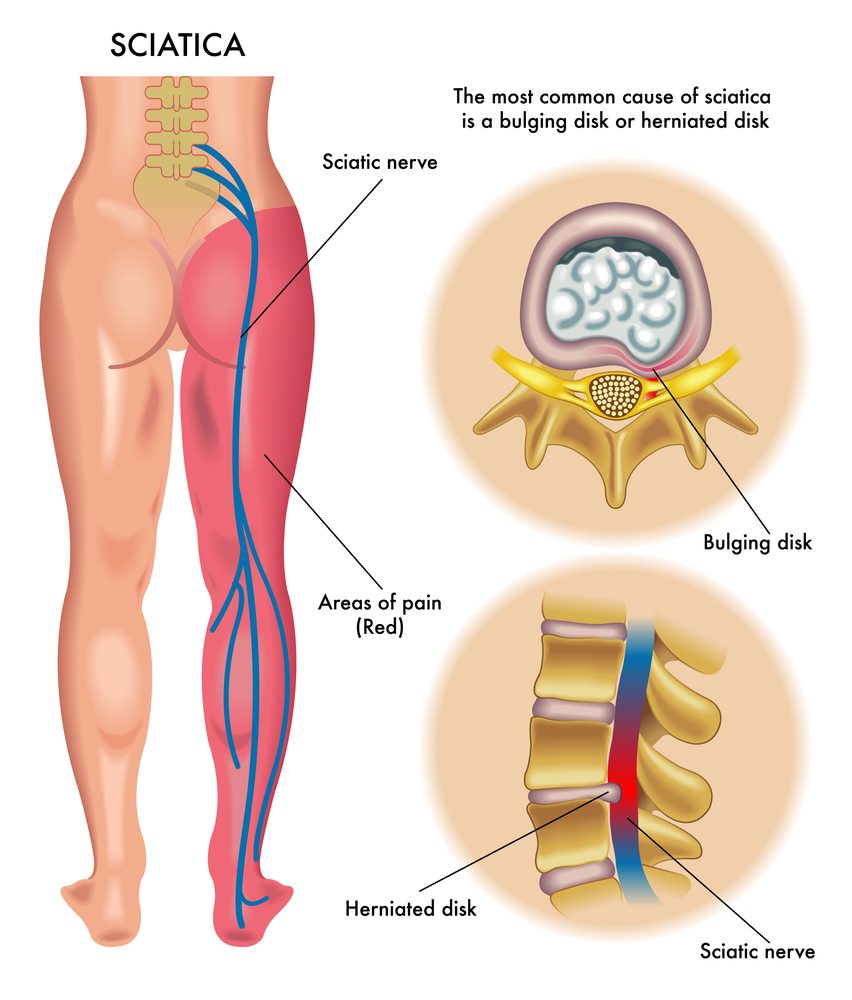
Sciatica
Doug Drysdale and Dr. Shaheen Lakhan join SpineThera Board – Valdosta Daily Times

PLYMOUTH, Minn.–(BUSINESS WIRE)–May 16, 2022–
SpineThera, Inc., privately held pharmaceutical company at the clinical stage that is focused on its development and testing of SX600 (a innovative formulation of dexamethasone that is sustained release for the treatment of sciatica) was announced on the 21st of May that its biopharma executive Doug Drysdale and physician-scientist Dr. Shaheen Lakhan have joined the SpineThera board of directors to assist in the continued research and development for SX600 and the expansion of SpineThera’s pipeline of product development.
“As SpineThera nears completion of the SALIENT clinical trial, we’re thrilled to welcome Doug as well as Shaheen become part of our Board of directors to guide the company in its future growth steps,” said Jeff Missling the CEO and Chairman. “Doug has an impressive background in executive management, fundraising and the process of making deals on our boards. In addition, Shaheen’s experience as a physician in neurology and pain and his executive leadership abilities in clinical development provide an unbiased medical perspective to the board. I am thrilled to have them on the of our team as we progress SX600 through the clinical development process for sciatica and also as we begin to make use of our micro-suspension technology to create an infrastructure.”
Doug Drysdale is the CEO at Cybin, Inc., an innovative biopharmaceutical company that focuses in advancing the use of psychedelics as therapeutics through the development of unique platform for drug discovery, innovative drug delivery systems, innovative formulation strategies and treatment regimens for mental health conditions. As a highly experienced corporate director and chief executive officer, Doug has chaired the board of directors for an NASDAQ-listed company, and in his role as CEO for 14 years, he has created and transformed four pharmaceutical businesses.
Doug’s thirty years in the field of healthcare, He has completed 16 acquisitions of corporations across three continents, and has raised $4 billion of private and public capital. In his 5.5 years as the CEO of Alvogen the company has grown from the beginning to earning $450 million in revenue in 35 countries. In the beginning of 2014, Doug was the leader in the recapitalization of the NASDAQ-listed Pernix Therapeutics, raising $65 million. In the first year after being appointed the CEO and Chairman, Doug increased the company’s overall valuation from $80 million up to $800 million. Under Doug’s direction, Pernix raised $465 million of capital. From November 2017 until the end of July in 2020 Doug served as a director and the CEO at Tedor Pharma, a family-owned contract manufacturing company. Doug’s efforts in reviving the company resulted in an increase of 60% in revenue in the year 2019. As the previous Head of M&A at Actavis Group, Doug led 15 corporate acquisitions, and raised nearly $3 billion in capital. Doug holds a bachelor’s diploma from the department of Microbial and Molecular Biology from the University of East Anglia in the U.K. and was named as the Entrepreneur of the Year in 2012 by Ernst and Young in 2012.
Drysdale declared, “There is a tremendous market opportunity for sciatica as there are 10 million epidural injections with steroid (ESIs) each year and there are no FDA approved medications available. SX600 is a potential solution to drastically reduce the necessity for repeated ESIs during the initial months of treatment as well as to provide long-lasting pain relief for patients. SpineThera’s technology for platform delivery is able to sustain the release of active substances for up to 90 days with a single injection made with tiny needles or catheters which opens up a variety of commercial opportunities as well as sciatica.”
The Dr. Shaheen Lakhan is a medical doctor, scientist and clinical developer who has more than 15 years of experiences in clinical practice, industry, and academia. He is board certified in both pain medicine and neurology with a clinical education from Cleveland Clinic in Cleveland and Massachusetts General Hospital. He is currently the Chief Medical Officer of Click Therapeutics where he is changing the way medicine is practiced and democratizing healthcare through digital therapies. Additionally, he continues to provide patients as an active neurologist and pain expert in the Cambridge Health Alliance. In the past Dr. Lakhan was faculty of renowned institutes (Harvard Medical School Case Western Reserve University) as the department’s chair and the curricular director (California University of Science and Medicine) and also as an chair of the institution’s review board (Arrowhead Regional Medical Center) Chief of the department for pain and rehabilitation (Virginia Tech Carilion Clinic), chief of pain management (Virginia Tech Carilion Clinic) and leadership and executive roles in biopharma (Zogenix, Sage Therapeutics) and biotech (Fern Health, The Learning Corp). He was awarded by the US President’s Call to Service Award for Lifetime Achievement by George W. Bush. He was praised by his peers. the award was conferred to him as a Fellow of the American Academy of Neurology, the Academy’s highest distinction.
“For patients, SX600 holds the potential to be the best-in-class treatment to ease the suffering and disability in patients suffering from chronic radicular pain. For the doctor in a field that is medicine lacking evidence-based, FDA-approved treatments, I would expect my colleagues in pain medicine are likely to include SX600 in their arsenal of pain-killers,” said Dr. Lakhan. “The shocking facts that more patients than not are unable to take their medication according to the prescription, and the fact that a third of prescriptions are never fulfilled. After being validated through the SX600 program, SpineThera’s unique drug delivery system can combat these staggering statistics by reaching that extends over spine programs and indications for pain.”
about SpineThera
SpineThera is a stage of clinical development pharmaceutical company, working to enhance the lives of patients by developing injectable medications using the micro-suspension platform technology. The SpineThera micro-suspension offers a long-lasting sustained release from the pharmaceutical active component that has high-quality injectability in extremely high levels. Our aim is to create unique drugs that give patients and doctors with innovative treatments that lower risks, enhance outcomes, and lower overall costs for treatment in the market with little or no approved medications. Our principal investigational product SX600 is a new formula of dexamethasone that is which is being developed for sciatica. SX600 is being evaluated in the Phase 2 demonstration of concept clinical trial. SpineThera, Inc., is located within Medical Alley, Minnesota, the epicenter of global healthcare innovation and research.
View source version on businesswire.com:https://www.businesswire.com/news/home/20220516005137/en/
Jeff Missling |[email protected]
Keyword: UNITED STRATES North AMERICA MINNESOTA
Keywords for the INDUSTRY: BIOTECHNOLOGY PHARMACE Health Clinical Trials
SOURCE: SpineThera, Inc.
Copyright Business Wire 2022.
PUB: 06:11 AM/DISC: 06:11 AM on 05/16/2022
http://www.businesswire.com/news/home/20220516005137/en

We understand how important it is to choose a chiropractor that is right for you. It is our belief that educating our patients is a very important part of the success we see in our offices.