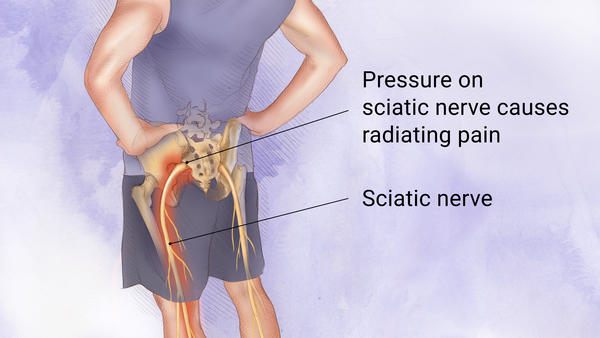
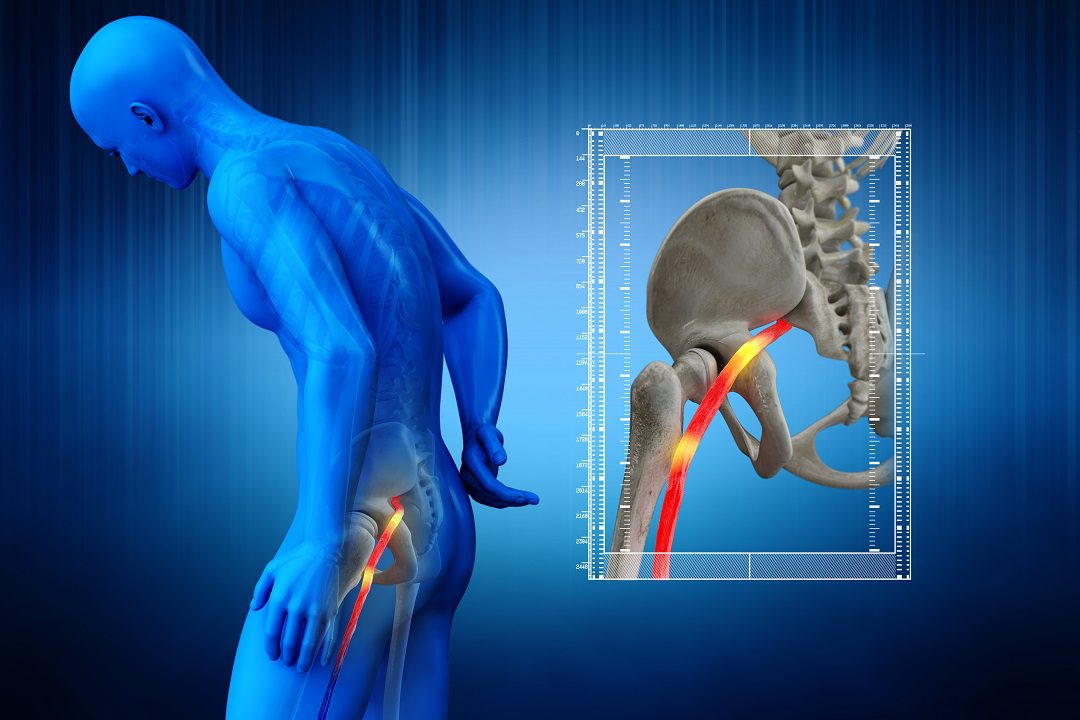
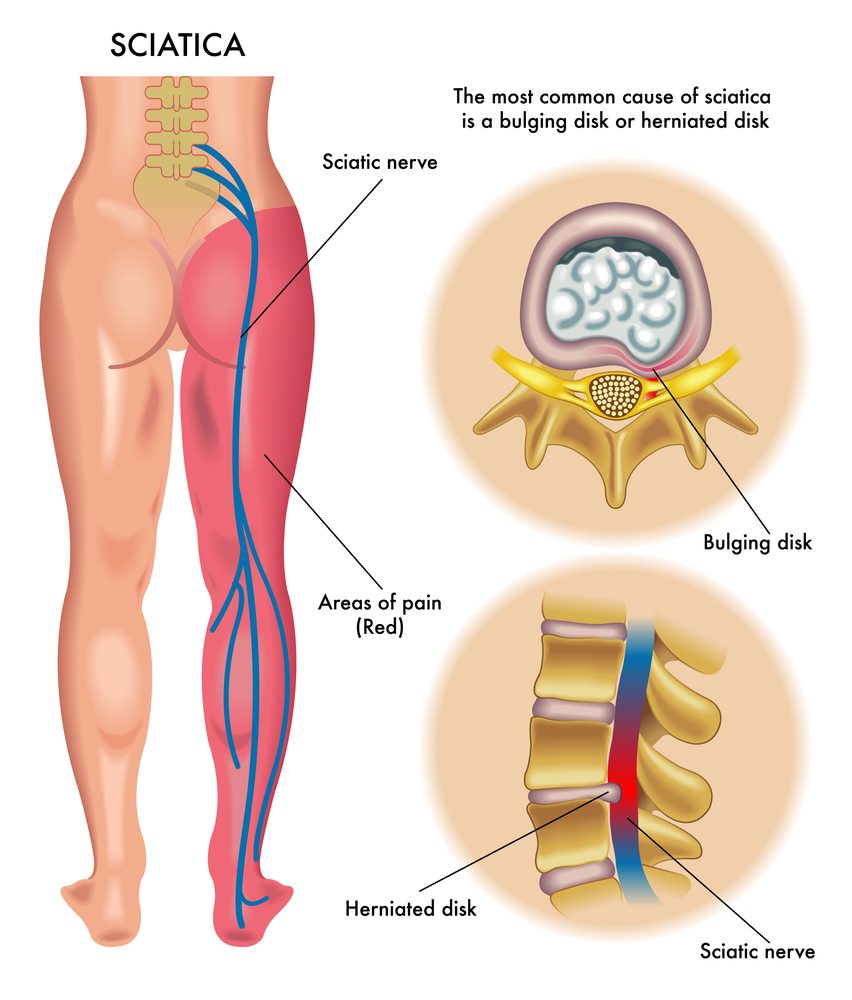
Sciatica
Innovative Sciatica Treatment Offers Significant Pain Relief phase 3 of the trial – Monthly Prescribing Reference
The final results were released from an investigation in phase 3 that tested SP-102 (injectable dexamethasone sodium viscous gel containing phosphate) for treatment of Lumbosacral radicular pain or sciatica.
SP-102 is a sodium dexamethasone viscous gel formulation of phosphate that is administered through epidural injection. The product under investigation is free of preservatives, surfactantsor solvents or particles.
3. The Phase III C.L.E.A.R study (ClinicalTrials.gov Identifier NCT03372161) The trial compared the analgesic effects and the safety of one and repeated transforaminal injections with SP-102 versus placebo. The study included 401 adult participants who were diagnosed with Lumbosacral Radicular Pain. The primary goal was to determine the change in mean leg pain scores by using the Numerical Pain Rating Scale, from baseline to week four. The other key endpoints were the change of the Oswestry Disability Index (ODI) score, as well as the duration of an open-label repeat injections.
After a single injection of SP-102, the results revealed a least square (LS) median (SE) group-wide difference that was -1.08 (0.17) to the variation in the average daily discomfort on the leg affected over four weeks when compared to the placebo ( P <.001). Patients who received SP-102 reported relief of pain beyond 4 weeks. The average time for repeat open-label treatment of 99 days (95 percent CCI of 78-129) According to the Kaplan-Meier’s estimation.
The treatment with SP-102 was linked to an increase of 28% in ODI score after 4 weeks, compared to the baseline. The average (SE) of the group’s difference of ODI score was found as -6.28 (1.49) when compared to placebo at week 4. ( P <.001).
Furthermore, statistically significant results were found for other secondary endpoints, such as Global Impression of Change, Brief Pain Inventory, PainDETECT and the cumulative use the medications to treat pain (acetaminophen).
In terms of security, there were not significant adverse events linked either to injection procedure and no adverse reactions reported of particular significance (eg hematoma or an infection of the site where injections were administered and paraplegia).
“We are extremely pleased by the results and the results of this trial are quite amazing,” said Dmitri Lissin, MD, Chief Medical Officer of Scilex. “We believe that SP-102 (SEMDEXA(tm)) is a promising drug with the potential to become an effective treatment for sciatica, an area that hasn’t had any significant advances in the treatments patients and doctors have been seeking for decades.”
The Food and Drug Administration (FDA) has previously approved Fast Track designation to SP-102 for this indication.
Reference
Scilex Holding Company, a majority-owned subsidiary of Sorrento Therapeutics, Inc. The company announces the final results of SP-102 (Semdexa(tm)) effectiveness and safety in its pivotal clinical trial in phase 3 of its program to manage sciatica pain which suggests the possibility of a first-to-market opportunities. News release. Sorrento Therapeutics, Inc. Accessed March 22, 2022. https://www.globenewswire.com/news-release/2022/03/18/2406054/0/en/Scilex-Holding-Company-a-majority-owned-subsidiary-of-Sorrento-Therapeutics-Inc-Announces-Final-Results-for-SP-102-SEMDEXA-Efficacy-and-Safety-from-its-Pivotal-Phase-3-Clinical-Tri.html

We understand how important it is to choose a chiropractor that is right for you. It is our belief that educating our patients is a very important part of the success we see in our offices.