

Fasting intermittently alters the intestinal bacteria activity of mice and improves their ability to heal from nerve injury. The research was released in Nature and was...
Check out this article Click Here to Experience this Innovative Method for Relieving Sciatica Pain You can do a variety of stretching exercises to help reduce...

I recently asked my readers about the top issues they face in relation to their condition of musculoskeletal. What issues were they searching for answers to...
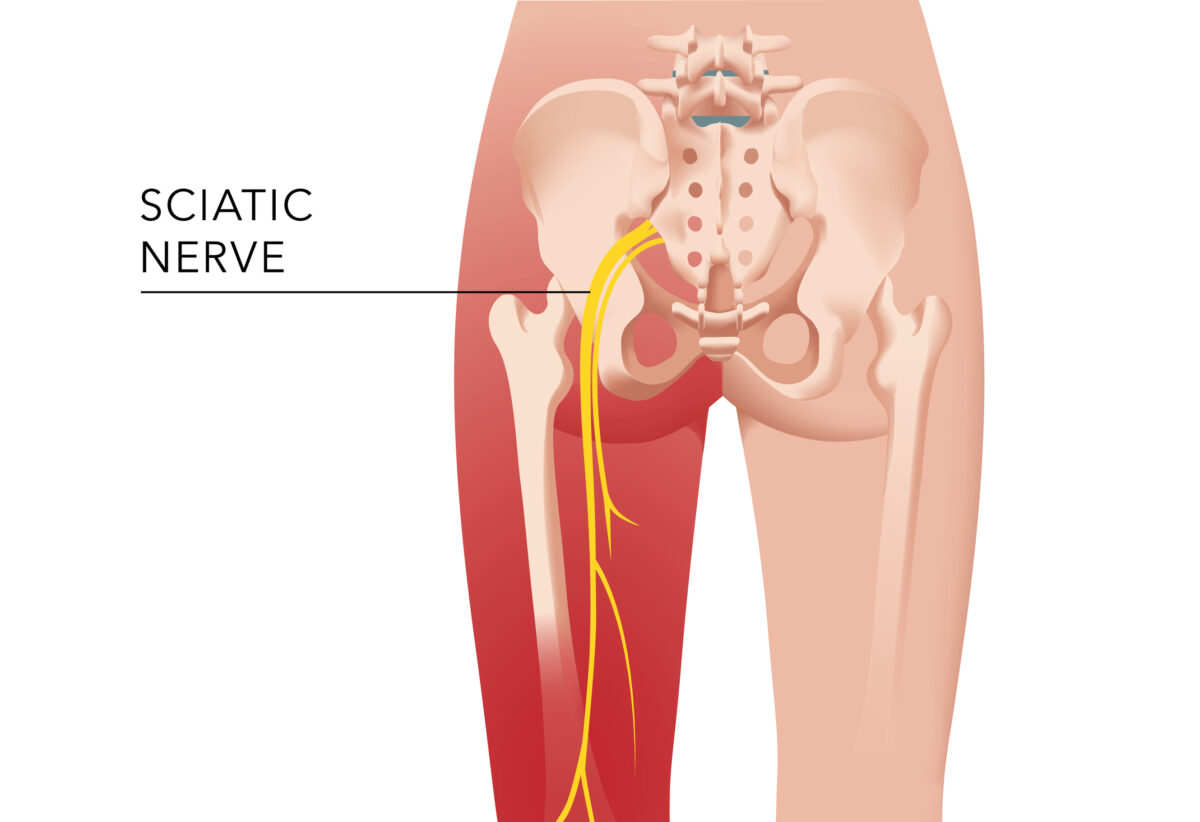

Our society is increasingly sedentary and this is especially true as we age. As we age the more difficult it is to stay from sitting for...


This article is part a ongoing series designed to elevate the voices of New York’s medicinal marijuana users. Subscribe to our NYCI newsletter here, or sign...


Pope Benedict XVI stepped down at age 85. He was the first pope to retire in the history of Gregory XII in 1415. There are now...
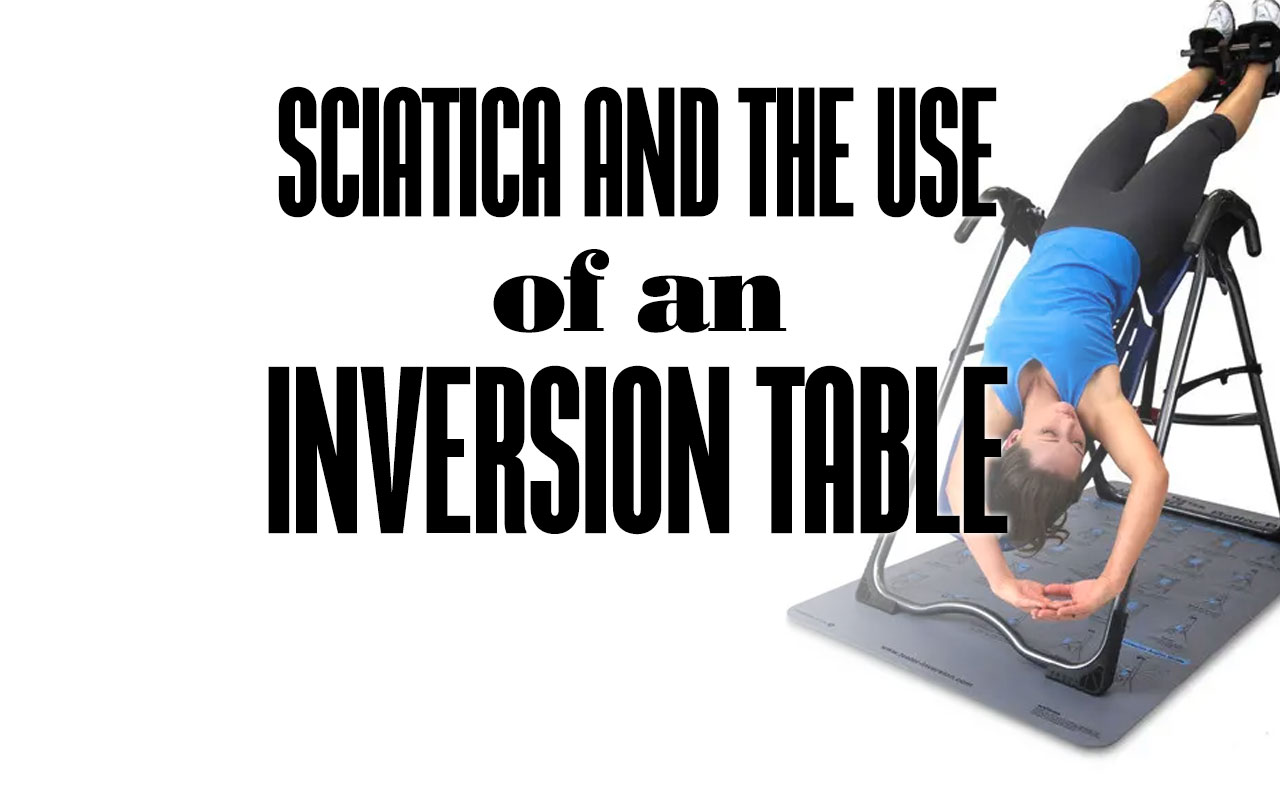

Inversion tables are devices that allow you to hang upside down or at an angle, to stretch and decompress your spine. Some people claim that inversion...

ROCHESTER, Minn. — I’m a dad. I share this trite fact as I recently completed the task of preparing our house to host the graduation ceremony...


In the moments before her life was shattered, Megan Freedman had attended an unforgettable business dinner attended by beloved colleagues at a fashionable dining establishment located...

Read this article Okay Where are my lazy ladies? As modern-day urban dwellers, the majority of us lead a very uninvolved lifestyle, a sluggish eating habits,...