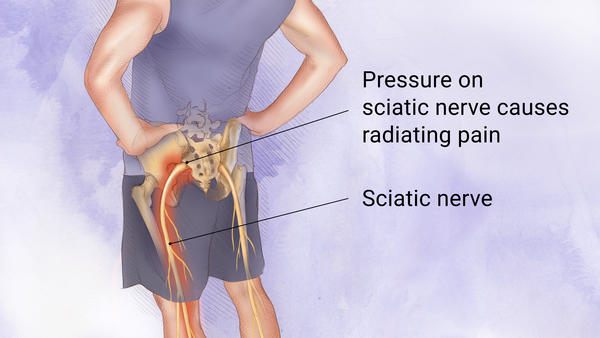
Sciatica
“Results are clear” Eliem’s lead program is ineffective and share prices halve before the bell goes off Endpoints News
As Eliem Therapeutics launched last March the its CSO Valerie Morisset told Endpoints News that when she was offered the chance to make a move into biotech her initial plan was to “basically end the company and move on towards the next one.” Instead she joined the Seattle-UK biotech and helped quickly launch the company five months following that shady withdrawal.
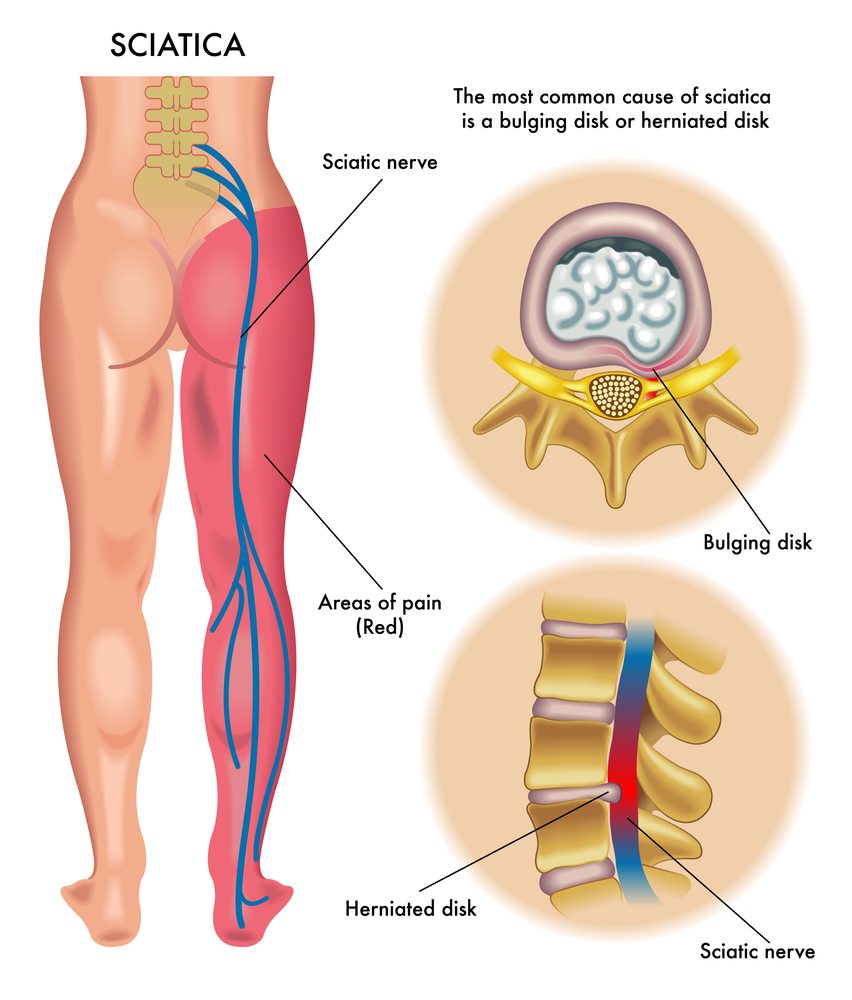
However, it seems Eliem is required to follow Morisset’s initial approach and proceed to the next project as the company’s principal program has been unsuccessful in a phase IIa clinical trial for patients suffering from chronic peripheral neuropathy (DPNP). The drug, ETX-810 will continue to be used for a second indication with Phase IIa results for the treatment of lumbosacral radicular discomfort, or sciatica, scheduled for the third quarter of.
It’s only one part of the double punch revealed Monday morning that sent Eliem’s shares $ELYM down 52% ahead of the bell rang at the beginning of the day.
The third obstacle to Eliem’s rapid rise is that the company has delayed the enrollment of a different medication, ETX-155, in Phase IIa trials across a variety of depression conditions due to evidence of pharmacokinetics from the Phase Ib study in patients suffering from epilepsy with photosensitivity.
“The inability to complete the ETX-810 DPN trial is disappointing as to ELYM shares it eliminates one of the major upside factors. But, we believe there’s a lot of hope for ELYM’s ETX-810 LSR/sciatica trial that will be finish reading in 2H22because sciatica is one of the indications that has the strongest evidence-based clinical case in the field of PEA,” Stifel analysts wrote in a report.
Eliem President and CEO Bob Azelby, in a press release, described these results in terms of being “unambiguous,” noting there is no path to development for ETX-810 in the DPNP. The asset is a prodrug of the bioactive lipid palmitoylethanolamide, or PEA.
“Given the favorable precedent research evidence supporting ETX-810’s mode of action across multiple neuromuscular pain groups, it’s disappointing that we were not able to verify a positive effect for DPNP patients who were part of this study. We believe that the entire aspects of the trial were properly executed, with proper choice of patients, well-balanced study arms and a placebo effect that was in line with expectations.” Azelby said.
The failure of DPNP was due to the prodrug’s inability effectively differentiate itself from placebo in the primary purpose, which was to show an improvement from week 1 to week 4 in the average weekly daily pain as measured through an assessment scale. Eliem’s hopes are now pinned towards the sciatica trial.
“We must note that, while we think there is evidence in the PEA research that supports the efficacy of both indications, there’s greater evidence of effectiveness in LSRP. It is also important to note that Eliem found a clear security profile of [‘810] in the DPNP study, which was in keeping with expectations,” Stifel analysts wrote.
The second part of the double-whammy news release is Eliem’s ETX-155 appears to have “lower-than-expected exposure to the drug” in the Phase Ib study of epilepsy patients who are photosensitive, Morisset said in the press announcement. This has caused Eliem to halt participation in its Phase IIa clinical trials that will test the drugs in patients with major depression and perimenopausal. The study has included three patients. assessed to date in the Phase Ib trial which has shown that the levels of the drug are “significantly lower than what was expected” due to the pharmacokinetic profile observed at the two Phase I trials.
“[T]he other, significantly more surprising surprise this morning is the confusion of ELYM’s photosensitive epilepsy study ph1b for ETX-155. We had previously viewed the trial as a catalyst of a moderate level (i.e. most likely positive however, it is more gradual) However, ELYM has decided to delay the next study (MDD/PMD) in ‘155 due to the fact that they found lower than expected drug exposures. While we await more information about this issue, it is hard to assess,” Stifel anaylsts wrote in their report.
Eliem is studying the “potential reasons behind the apparent differences in the exposure of previous studies, and also the evaluation of possible differences in the batches of drug products utilized in this study and the ones used in previous Phase 1 trials,” the biotech stated.
The biotech has stated that it is “well capitalized to support the business through a variety of additional factors.” Eliem “does not plan to lay off employees at this point,” a company spokesperson said to Endpoints via email saying that the biotech has around 30 full-time employees.

We understand how important it is to choose a chiropractor that is right for you. It is our belief that educating our patients is a very important part of the success we see in our offices.